Recherchez sur le site !
Recherche avancée / SpécifiqueCatégories publications
+ Sciences De La Terre - Archéologie - Astronomie - Spéléologie - Ecologie - Pédologie - Volcanologie - L'hydrogéologie - Géomorphologie - Minéralogie - Pétrologie - Paléontologie - Géologie + Climatologie - Réchouffement climatique - Changement climatique + Plantes - Plantes Aromatiques - Plantes médicinales + Zoologie - Faunes + Botanique - Flors + Sciences humaines - Géo Eco Tourisme - L’anthropologie - L'Histoire - Démographie - Sociologie - Géographie - Patrimoine culturel
Géo éco tourisme inclusif

Géoparc et Recherche Scientifique
Le coins de l’étudiant



Blog Géoparc Jbel Bani
Subacute toxicity, anti-inflammatory and antioxidant activities of ethanolic extract of Moroccan Warionia saharae from Tata region (Géoparc Jbel Bani)
By FATIMA AMEZOUAR, WADI BADRI, MOHAMMED HSAINE, MOHAMMED AKSIM, NOUREDDINE BOURHIM, HASSAN FOUGRACH
Laboratoire d’Ecologie et Environnement, Université Hassan II-Mohammedia, Faculté des Sciences Ben M'sik, Avenue Cdt. Driss El harti
BP. 7955, Casablanca 20800, Maroc. Service central d’anatomie pathologique. CHU Ibn Rochd, Casablanca, Maroc. Laboratoire de Biochimie et Biologie Moléculaire, Université Hassan II-Aïn Chock, Faculté des Sciences, km 8 route d’El Jadida BP. 5366 Mâarif, Casablanca, Maroc Email: amezouarfatimazahra@ymail.com
Received: 02 Oct, 2012, Revised and Accepted: 03 Nov, 2012
In the present study, 30-day subacute toxicity, antioxidant and anti-inflammatory properties of ethanolic extract of Warionia saharae leaves were investigated. We have demonstrated the antioxidant and free radical scavenging abilities by using different in vitro bioanalytical methods (DPPH, PPM and FRAP). The IC50 value of W. saharae of saponins extract was weak (IC50 = 74.14 μg/ml), since its antioxidant activity was higher than that of ethanolic extract (IC50 = 182 μg/ml) and ethyl acetate extract (IC50 = 197.4 μg/ml). Histopathological examination and biochemical analysis of renal and liver functions, demonstrated that ethanolic extract at oral dose of 5 g/kg BW inducted nephrotoxicity and caused the damaged structural integrity of the liver. The ethanolic extract at doses of 200 and 400 mg/kg BW decreased the inflammation (56.42% and 61.45% inhibition, respectively) form the 2th h after carrageenan administration. Ethanolic extract has shown significant (p < 0.05) inhibition of paw edema when compared with control rats (NaCl 0.9%) and were higher to that of In the present study, 30-day subacute toxicity, antioxidant and anti-inflammatory properties of ethanolic extract of Warionia saharae leaves were investigated. We have demonstrated the antioxidant and free radical scavenging abilities by using different in vitro bioanalytical methods (DPPH, PPM and FRAP). The IC50 value of W. saharae of saponins extract was weak (IC50 = 74.14 μg/ml), since its antioxidant activity was higher than that of ethanolic extract (IC50 = 182 μg/ml) and ethyl acetate extract (IC50 = 197.4 μg/ml). Histopathological examination and biochemical analysis of renal and liver functions, demonstrated that ethanolic extract at oral dose of 5 g/kg BW inducted nephrotoxicity and caused the damaged structural integrity of the liver. The ethanolic extract at doses of 200 and 400 mg/kg BW decreased the inflammation (56.42% and 61.45% inhibition, respectively) form the 2th h after carrageenan administration. Ethanolic extract has shown significant (p < 0.05) inhibition of paw edema when compared with control rats (NaCl 0.9%) and were higher to that of diclofenac (reference drug). The present study supported the utilization of W. (reference drug). The present study supported the utilization of W.
Saharae in traditional Moroccan medicine and suggests that this plant present interesting potential source of natural antioxidants that could be used to treat inflammatory diseases.
INTRODUCTION
The use of traditional medicine is widespread and plant still represents a large source of natural antioxidants that might serve as leads for the development of novel drugs1. In the Moroccan traditional medicine, the leaves of Warionia saharae are used to treat inflammatory diseases, such as rheumatoid arthritis, for gastrointestinal disorders and epileptic crisis2. This species known by the vernacular names of “Afessas”, “Abessas” and “Tazart n-îfiss” is considered to have medicinal properties mainly by its essential oils3. The chemical composition of W. saharae leaves has been investigated and thus twelve new guaianolide type sesquiterpene lactones were identified. Cytotoxic and anti-inflammatory sesquiterpene lactones effects were showed4-6. The chemical composition of the hexanoic extract prepared using Soxhlet apparatus from W. saharae leaves was reported by Elamrani et al7.
In addition, several studies reported the chemical composition of W. saharae essential oil from the leaves8-11.
The genus Warionia has been recently transferred to the Cichorieae tribe by Katinas ET al12 based on molecular data. This monotypic genus was included in the Cichorioideae subfamily of Asteraceae by Bremer13 but was not assigned to any of its tribes. Warionia yielded sesquiterpene lactones and is therefore included in this review. Warionia saharae Benth. & Coss., is endemic to northwestern edge of the African Sahara desert, in Morocco; including Ijoukaka (High Atlas), Cape Ghir, Tamanar, Tazerwalt, Tiznit, Agadir, Ifni, Bou Izakarn, Oued Ouarksiz, Figuig and Erfoud, and in Algeria at Béni-Ounif (South Oran)12.
The present study was carried out to investigate the antioxidant capacity, histopathological toxicity and anti-inflammatory activity of the ethanolic extract of dried leaves of W. saharae in experimental rat model. Thus to obtain information on pharmacological properties and biosafety of leaves extract of this plant used in Moroccan traditional medicine.
MATERIALS AND METHODS
Plant material
Aerial parts of W. saharae (Warionia saharae Benth. & Cosson, Bull.Soc. Bot. France 19: 166. 1872) were collected from Tata region (650 Km south of Casablanca) in April 2010 and the biological authentication was carried out by professors Fougrach Hassan and Mohammed Hsaine in the Ecology and Environment Laboratory, Faculty of sciences Ben M’sik, University Hassan II-Mohammadia.
Chemicals
Folin-Ciocalteu Reagent (FCR), Butylated hydroxytoluene (BHT), Diclofenac (DIC) and vitamin E were purchased from Sigma Chemical Co. (St. Louis, MO), 2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, quercetin, carrageenan and vitamin C were obtained from Somaprol (Casablanca-Morocco), the biochemical assay kits were from BioSystems S.A. (Barcelona, Spain) and all other chemicals were of analytical grade.
Phytochemical screening
The phytochemical analysis of W. saharae was performed using the methodologies described by Wagner ET al14, Evans15, Cannell16 and Barbehenn17. The extracts of leaves W. saharae were tested for the presence of flavonoids (cyanidine reaction), polyphenols and tannins (FeCl3 test), alkaloids (Dragendorff’s reagent), sterols (Liebermann–Burchard test), saponins (frothing test), coumarins (visualization of the chromatogram under UV light 365 nm) and mucilage (precipitated by addition of absolute ethanol).
Preparation of extracts
Ethanol extract
The powder of dried leaves W. saharae (50 g) was extracted with absolute ethanol at room temperature (2×300 ml) for 24h.
Following the filtration, the ethanol extract was evaporated to dryness under reduced pressure at 45 °C. The extract yield was 8.90 ± 0.79% (w/w).
Ethyl acetate extract
50 g of powdered leaves were macerated for 4 h at 90 °C in 200 ml of ethanol/water (v/v); then the filtrate was concentrated under reduced pressure at 45 °C to remove the ethanol phase. The residue was extracted three times with ethyl acetate. The ethyl acetate phases obtained were combined and evaporated to dryness and conserved at 4 °C until use. The extract yield was 4.04 ± 0.13% (w/w).
Saponins extract
Saponins were extracted according to the method of Applebaum ET al18. The organic phase, evaporated at 45 °C, were weighed and stored at 4 °C for further studies. . The extract yield was 2.04 ± 0.03% (w/w). Each extraction was done in duplicate.
Dosage of total phenolic and total flavonoid compounds Extract of total phenolic and total flavonoid compounds was prepared from W. saharae powder as described by Chitindingue ET al19 slightly modified. The powder leaves (2 g) was extracted twice with 20 ml of aqueous methanol solution (50%). The two volumes were combined, made up to 40 ml, centrifuged at 4500 rpm for 20 min and stored at 4 °C in the dark for analysis.
Total phenolic compounds were determined following the method adapted from Wolfe ET al20, using Folin–Ciocalteu reagent. Gallic acid was used as a standard for the calibration curve. The total phenolic content was expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g DW). Total flavonoid compounds were measured according to a colorimetric assay of Zhishen ET al21. Total flavonoid content in leaves extract of W. saharae was expressed as milligrams-quercetin equivalents per gram of dry weight (mg QE/g DW). Extracts were analyzed in three replications.
Antioxidant activity
DPPH radical scavenging assay
The radical scavenging abilities of W. saharae were evaluated according to the slightly modified method of Pothitirat et al22. 0.5 ml of various concentrations of extracts and standards BHT, vitamin C and vitamin E separately were added to 1 ml of a 100 μM methanol solution of DPPH. The reaction mixture was incubated in the dark at room temperature for 20 minutes. The absorbance was monitored at 517 nm against blank containing methanol. The decrease in optical density of DPPH on addition of test samples in relation to the control was used to calculate the antioxidant activity as percentage of inhibition (%IP) of DPPH radical:
% IP = {(Abscontrol – Abssample)/Abscontrol} × 100. Where; Abscontrol is the absorbance of DPPH radical + methanol and Abssample is the absorbance of DPPH radical + sample extract or standard. The antioxidant activity of samples was expressed as IC50 in μg/ml required inhibiting the formation of DPPH radicals by 50%.
Determination of total antioxidant capacity
The total antioxidant capacity of ethanolic extract was evaluated by the phosphomolybdenum method (PPM) according to the procedure describe by Prieto ET al23. The assay is based on the reduction of Mo (VI)–Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at acid pH. A 0.1 ml extract was combined with 0.3 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes containing the reaction solution were incubated at 95 °C for 90 min.
Then the absorbance of the solution was measured at 695 nm against blank after cooling to room temperature. Ethanol (0.1 ml) was used as blank instead of extract and calibration curve was made using vitamin E as standard. The antioxidant capacity was expressed as the number of milligrams equivalents of vitamin E per gram of dry weight (mg EVE/g DW). Tests were performed in three replicates.
Reducing power assay
The FRAP (Ferric Reducing Antioxidant Power) of the ethanolic extract was determined according to the method of Oyaizu24. Various concentrations of the extracts (0 – 1000 μg/ml) in distilled water were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and 1% of potassium hexacyanoferrate water solution (2.5 ml, K3 [Fe (CN) 6]).
The mixture was incubated at 50 °C for 20 min. Aliquot of trichloracetic acid (2.5 ml, 10% aqueous solution) was added to the mixture which was then centrifuged at 3000 rpm for 10 min. The supernatant (2.5 ml) was mixed with distilled water (2.5 ml) and a freshly prepared FeCl3 solution (0.5 ml, 0.1%). The absorbance was measured at 700 nm. Vitamin E was used as a positive control. In this method, higher absorbance indicates higher reducing power of the extract.
Pharmacological procedures
Animals
Albino Wistar rats of either sex weighing 190 ± 50 g body weight (BW) were used in the present study. These animals were raised in the animal house of the Faculty of sciences Ben M’sik, University of Hassan II-Mohammedia. They were kept under controlled room temperature allowing food and water ad libitum and were exposed to 12 h day/night cycles. All experiments were carried out in compliance with the Guide for The Care and Use of Laboratory Animals.
Subacute toxicity
Animals were divided into five groups of six animals (3 males and 3 females) for each dose level of ethanolic extract of W.
saharae and control group. Subacute toxicity was evaluated after oral single daily administration (10 ml/kg BW) of ethanolic extract at 0.5, 1.25, 2.5 and 5 g/kg BW for a period of 30 days.
The control group received distilled water. Toxic manifestations and mortality were monitored daily, whereas body weight changes were recorded every 5 days.
Biochemical analysis
At the end of the 30-day period, all surviving animals were fasted overnight, anesthetized with diethyl ether and sacrificed by decapitation afterwards for blood collection from a common carotid artery at the neck. The blood collected was allowed to clot before centrifugation (4000 rpm at 4 °C for 5 min) to obtain serum, which was analyzed for glucose, creatinine, aspartate transaminase (AST), alanine transaminase (ALT) and total cholesterol.
Histopathological examination of the liver and kidney tissues
After blood collection, liver and kidneys were carefully dissected out.
Small slices of these freshly harvested tissues were fixed in formalin solution (10%), dehydrated by serial ethanol solution, diaphanized with ethanol-toluene and enclosed with paraffin. Micrometer sections, cut by a rotary microtome, were stained with hematoxylineosin
(H&E) and examined under a light microscope.
Photomicrographs of the samples were recorded.
Carrageenan-induced hind paw edema model
Anti-inflammatory activity was measured using carrageenaninduced hind paw edema assay according to the method of winter et al25 and Adeyemi et al26. 30 min after intramuscular administration of the ethanolic extract, each rat was injected into subplantar tissue of the right hind paw with 0.2 ml of a freshly prepared 1% carrageenan suspension in physiological
Anti-inflammatory activity was measured using carrageenaninduced hind paw edema assay according to the method of winter et al25 and Adeyemi et al26. 30 min after intramuscular administration of the ethanolic extract, each rat was injected into subplantar tissue of the right hind paw with 0.2 ml of a freshly prepared 1% carrageenan suspension in physiological saline. Experimental rats were divided randomly into five groups with three rats in each group. Group A; rats were used as untreated control. Each rat of the Group B received saline (10 ml/kg) only. Each rat in Group C and D received 200 and 400 mg/kg BW of W. saharae ethanolic extract respectively. Group E; positive control rats received DIC as reference drug (100 mg/kg BW). Linear diameter of the injected paw was measured at 0 h (D0: before phlogistic agent injection) and 1, 2, 3, 4, 5 and 24h intervals later (Dt). Percentage inflammation (edema) was calculated from the formula: (Dt - D0)/D0×100, while percentage inhibition of the edema was calculated from the formula: [(Dt - D0) control - (Dt - D0)treated] /(Dt - D0)control ×10027.
Statistical analysis
In each test, the experimental data represent the mean ± Standard Deviation (SD). The IC50 was obtained with a computer program GraphPad (Prism V. 5.00) by plotting the percentage of inhibition versus the logarithm concentrations. Statistical analyses were performed with XLSTAT V.7.1. The significance of the difference among groups was analyzed using ANOVA followed by the Fisher (LSD test) and Dunnett tests. Differences were considered significant at the level p < 0.05.
RESULTS
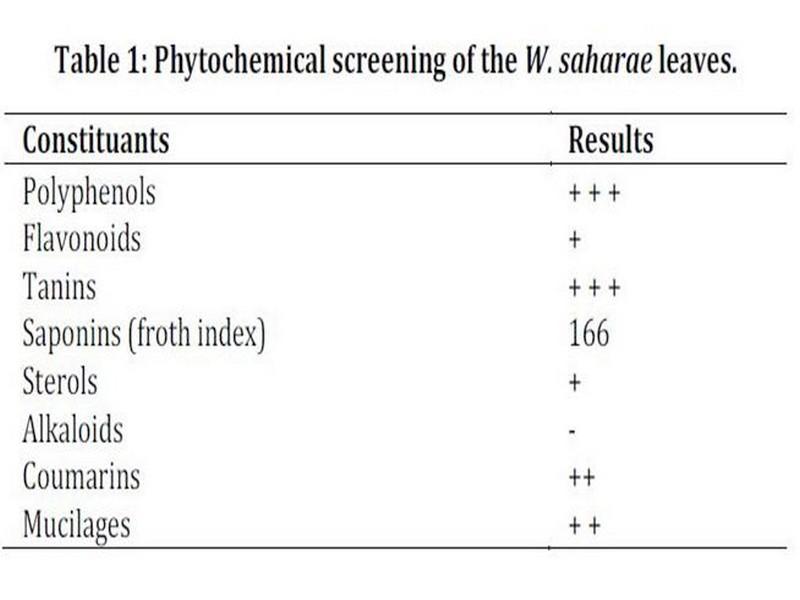
phytochemical screening, phenolics and flavonids contents The result of the phytochemical screening is presented in Table 1.
This reveals abundant presence of polyphenols and tannins, moderate presence of saponins, coumarins and mucilages and a weak concentration of flavonoids and sterols. Test was negative for alkaloids.
Table 1: Phytochemical screening of the W. saharae leaves.
+ + +: Abundant, ++: moderately presence, +: weakly present, -: absent.
The amount of total phenolics and flavonoids measured according to the methods described previously showed that their contents were 57.85 ± 0.03 mg EAG/g DW and 15.95 ± 0.01 mg EQ/g DW respectively. These results are in agreement with recent data for the same species from Algeria28.
Antioxidant activity
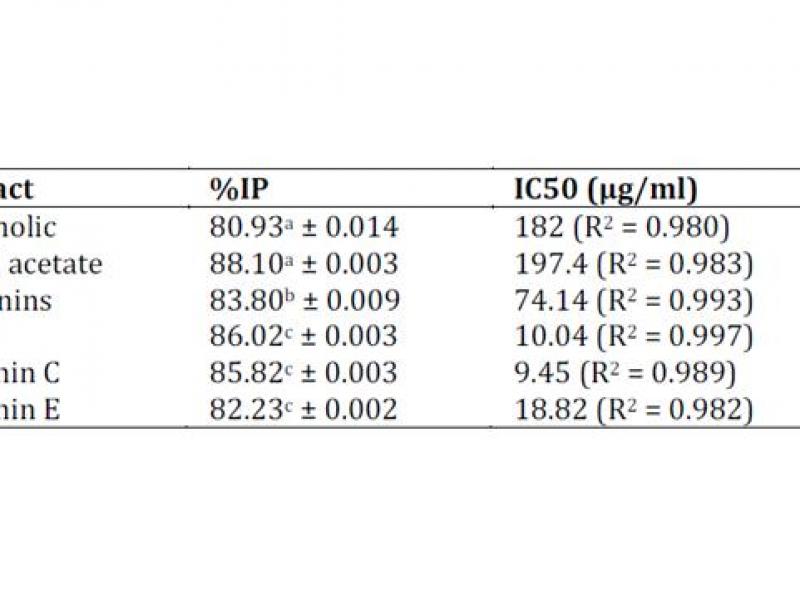
The antioxidant activities of W. saharae were compared with the standards vitamin C, BHT and vitamin E. DPPH free radical scavenging activity (%IP) was evident for all extracts tested as showed in Table 2.
A lower IC50 value indicates a greater antioxidant activity. The IC50 value of W. saharae of saponins extract was weak (IC50 = 74.14 μg/ml), since its antioxidant activity was a higher than that of ethanolic extract (IC50 = 182 μg/ml) and ethyl acetate extract (IC50 = 197.4 μg/ml) (Table 2). These results reveal that extracts of W.
Saharae have important antioxidant properties. That was confirmed by FRAP test measuring the reductive ability and PPM test of ethanolic extract. The values of FRAP and PPM tests were 20.15 ± 0.037 mg EVE/g DW and 39.29 ± 0.028 mg EVE/g DW respectively.
Table 2: DPPH scavenging activity of W. saharae extracts.
Values represent mean ± SD for three replicates.
IP = Inhibition percentage, BHT = Butyl Hydroxyl Toluen. a1000 μg/ml, b600 μg/ml, c100 μg/ml.
Subacute toxicity
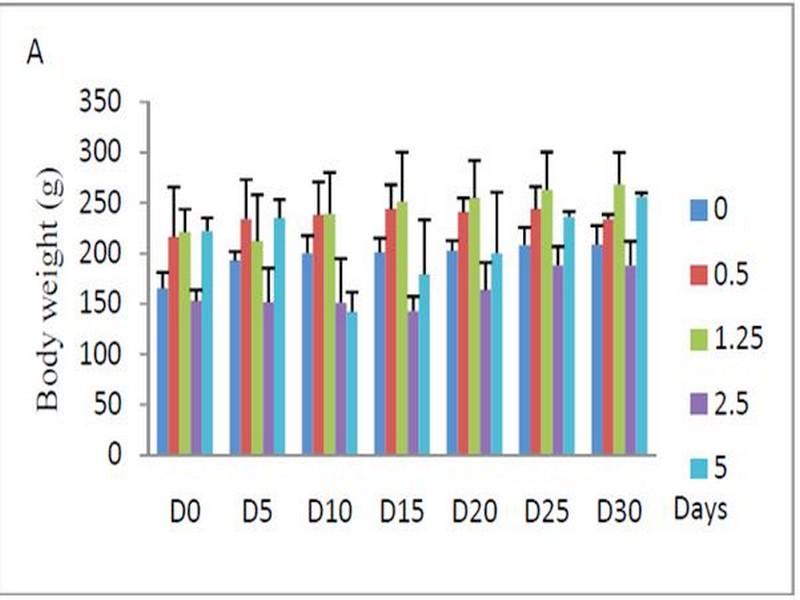
Oral administrations of doses 0.5 to 2.5 g/kg BW of W. saharae ethanolic extract were found to be safe in rats during the 30-day treatment period. The body and liver weight of the animals given ethanolic extract daily did not show any significant differences compared with the control group (Table 3). However, the dose 5 g/kg BW of the plant extract presented signs of toxicity and caused death of animals. In this group we marked a swelling of the kidneys and the liver weight was higher than that of the control group as presented in Table 3.
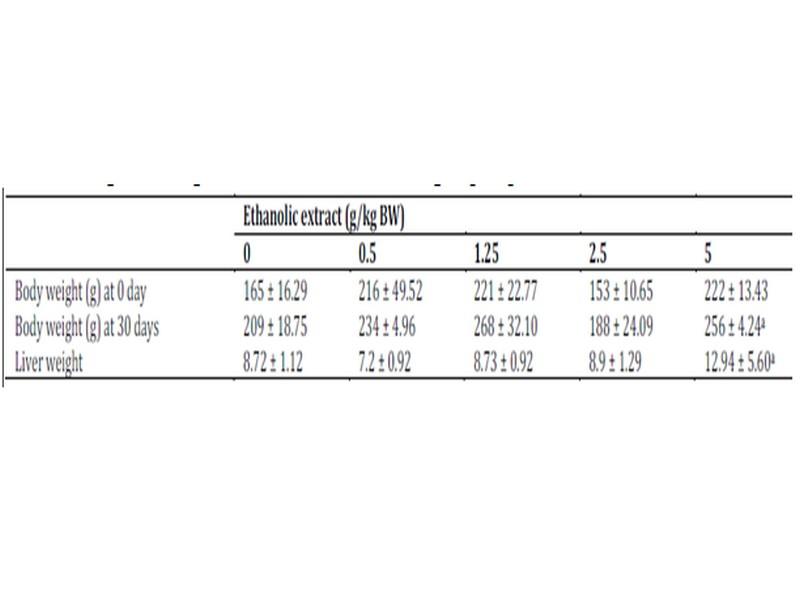
Data are expressed as mean ± SD, n = 6. aValues are the means of tow surviving rats. No statistical difference between the control (10 ml/kg BW of distilled water) and ethanolic extract W. saharae treated group (P < 0.05).
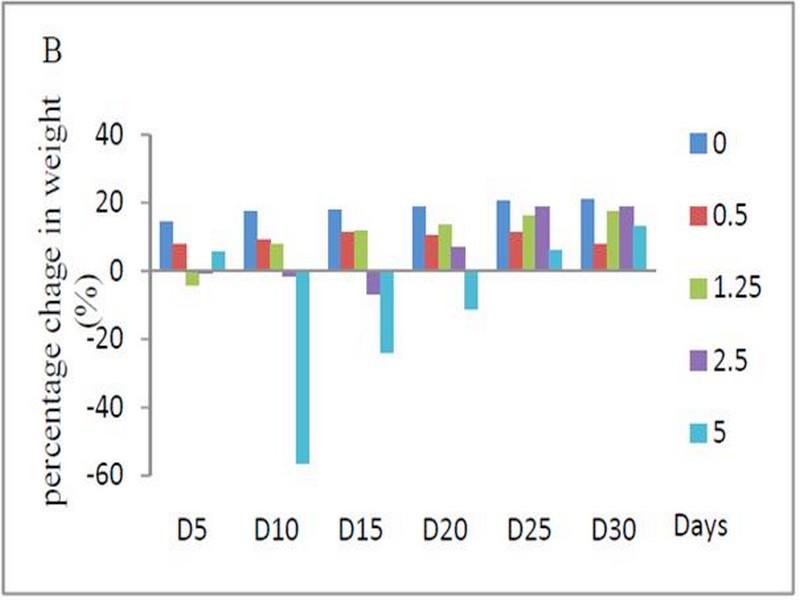
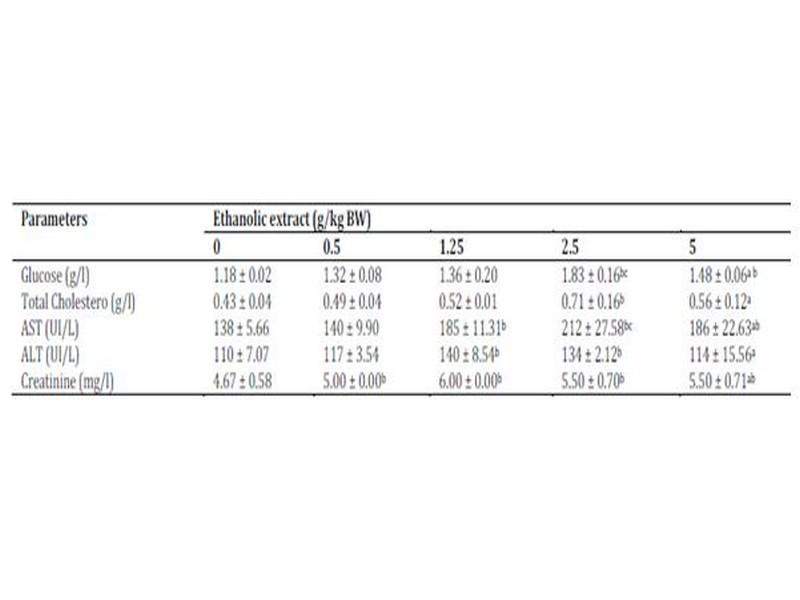
Signs of toxicity and deaths were recorded during the 30 days of treatment via oral administration of ethanolic extract. 66 % of the animals treated with oral daily dose of 5 g/kg BW of ethanolic extract were died. The LD50 value of the ethanolic extract of W. saharae toward Artemia salina was determined in our precedent work and it was found to be 45.02 mg/ml11. According to the classification of Meyer et al29, the ethanolic extract of leaves of W. saharae can be classed as not toxic. The percentage weight change in the groups treated with 0.5, 1.25 and 2.5 g/kg doses daily (Figure 1) tended generally to be higher compared to control group. The group received 5 g/kg BW marked a decrease of body weight until 21 days of period treatment (-56%, -24%, -11% at 10th, 15th and 20th days respectively). After that, all male rats and one female were died in this group. The two females which succeeded in adapting to the treatment have started to gain weight (5% and 13% at 25th and 30th days respectively). A study of biochemical parameters showed that the ethanolic extract treatment induced a significant (p < 0.05) increase of glucose serum at the doses of 2.5 and 5 g/kg BW when compared to the control group. Total cholesterol showed a significant increase at the dose of 2.5 g/kg BW. The activities of the enzymes ALT and AST and concentration of serum creatinine were significantly increased in the groups treated with 1.25, 2.5 and 5 g/kg BW compared to the control group (p < 0.05) (Table 4).
Fig. 1: (A) Effect of subacute oral administration of ethanolic extract of W. saharae on the body weight of rats (n = 6) for 30 days at the following doses: 0 (control), 0.5, 1.25, 2.5 and 5 g/kg BW. The rats were weighed before the start treatment (D0) and then every 5 days (D5, D10, D15, D20, D25 and D30). (B) Percentage change in the weight of rats compared to the pre-treatment values during 30-day of subacute treatment. In the 25th and 30th days, the values are the means of tow surviving rats.
Table 4: Biochemical parameters of rats treated with ethanolic extract of W. saharae.
Data are expressed as mean ± SD, n = 6. aValues are the means of tow surviving rats. All groups were administered at 0.5, 1.25, 2.5 and 5 g/kg BW daily for 30 days. Significantly different from the control group (0 g/kg BW) (P < 0.05): bFisher test, cDunnett test.
Histopathological study
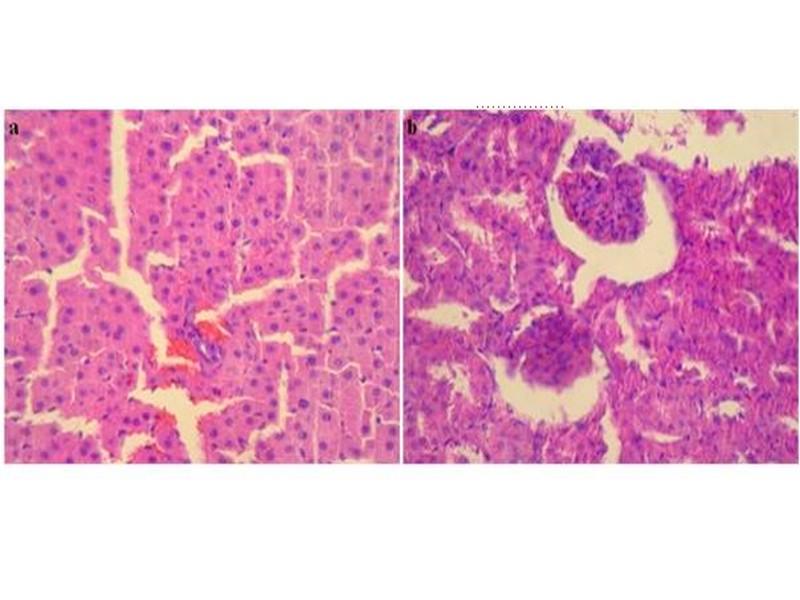
Light microscopic examination did not reveal changes in control group (Figure 2) and the treated groups with following daily doses: 0.5, 1.25 and 2.5 g/kg BW of leaves ethanolic extract of W. saharae. However, histopathological changes were seen in the group received 5 g/kg BW.
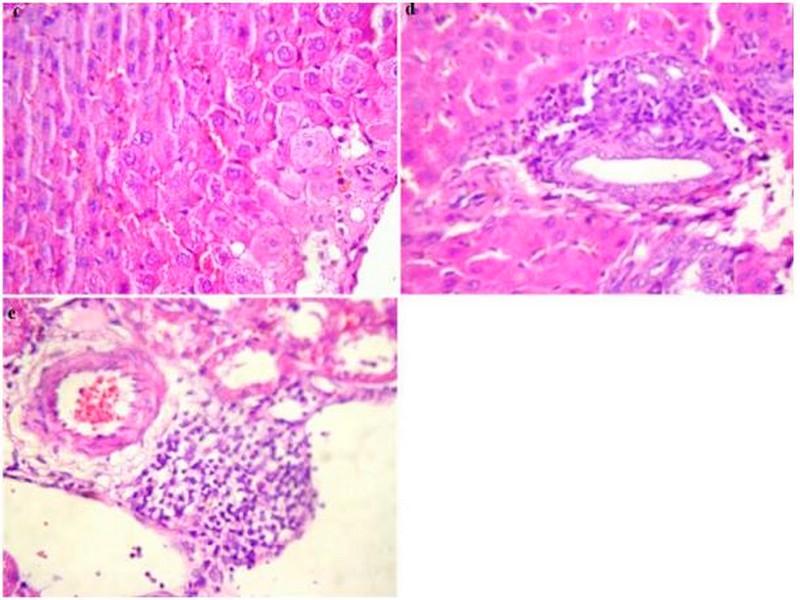
These findings in liver and kidneys are elucidated in Figure 3. An enlargement of the cell size was noticed in liver tissue. Cytoplasm of hepatocytes was light and seat of cholestase with sinusoidal congestion (Figure 3c). Inflammatory infiltrate portal made up of lymphocytes and rare polynuclear eosinophils (Figure 3d) was also evident in all surviving animals of the ethanolic extract group at the dose of 5 g/kg BW. The kidneys morphology of this group was marked by an infiltration of nodular lymphoid in the renal interstitium (Figure 3e). Treatment with the ethanol extract of W. saharae at dose of 5 g/kg BW demonstrated toxic manifestations in the liver and kidneys in the experimental animals.
Anti-inflammatory effect
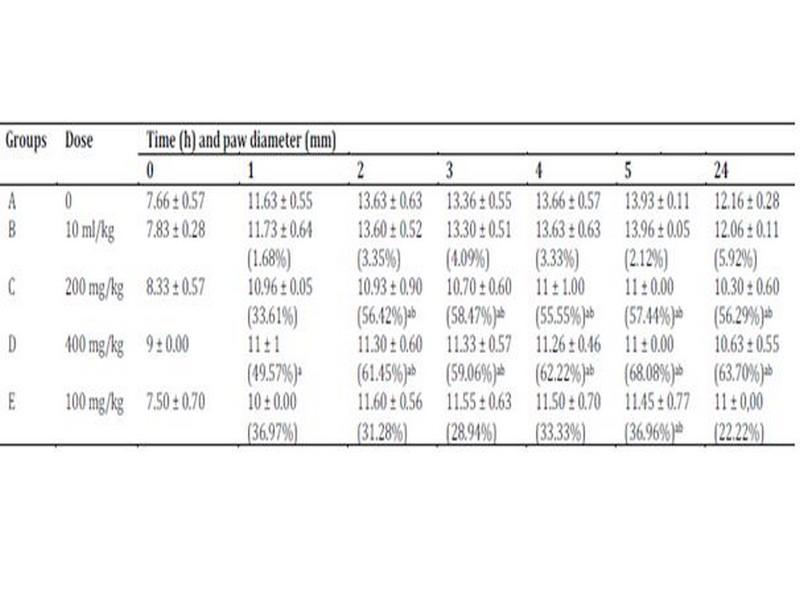
Carrageenan-induced hind paw edema model was used to evaluate the anti-inflammatory properties of ethanolic extract of W. saharae leaves. The subplantar injection of carrageenan (1%) produced a local edema that increased progressively to reach its maximum in 5 h (81.73 ± 0.11%). The extract decreased significantly the carrageenan-induced increase in paw diameter as compared with control rats (Table 5). One hour after carrageenan administration, ethanolic extract of W. saharae at doses of 200 and 400 mg/kg BW showed a potent inhibition of edema formation which were 33.61 ± 0.05% and 49.57 ± 1% respectively, while that of control (group B) was 1.68 ± 0.64%. The inhibition in edema formation reaches maximum significant values at 5 h after treatment (57.44% and 68.08% respectively at the indicated doses) and was higher to that of the non-steroidal drug diclofenac (36.96 ± 0.77%).
Fig. 2: Photomicrographs of normal hepatic parenchyma (a) and normal kidneys parenchyma (b) from control rat (H&E, x 400).
Fig. 3: Histopathological changes in the liver and kidneys in rats group treated orally with 5 g/kg BW of ethanolic extract of W. saharae for 30 days (H&E, x 400). (c) Enlarged hepatocyte sizes, Light cytoplasm and seat of cholestase with sinusoidal congestion. (d) Inflammatory infiltrate portal made up of lymphocytes and rare polynuclear eosinophils. (e) Infiltration of nodular lymphoid in the renal interstitium.
Table 5: Anti-inflammatory activity of ethanolic extract of W. saharae (200 and 400 mg/kg BW) and DIC (100 mg/kg BW) on rat hind paw edema induced by carrageenan.
Data are represented as mean ± SD (n=3). Percentage inhibition of the carrageenan-induced inflammation by the W. saharae ethanolic extract and the reference drug (DIC) are indicated as (%). Significantly different from the control group B (NaCl 0.9%) (P < 0.05): aFisher test, bDunnett test.
DISCUSSION
This study was conducted to evaluate the antioxidant, the subacute toxicity and the anti-inflammatory properties of W. saharae leaves which were widely used in traditional medicine in south of Morocco.
The phytochemical screening of the chemical composition of extracts showed the presence of secondary metabolites in leaves of W. saharae such as polyphenols, tannins, flavonoids, saponins and caumarins. We investigated the antioxidant capacity with DPPH radical scavenging; PPM test and reducing power assay (FRAP) which reflects the electron donating capacity of bioactive compounds in extract of W. saharae leaves. The DPPH radical is a free radical, which has been widely used as a tool to estimate free radical scavenging activities of several natural compounds such as phenolics and anthocyanins or crude mixture such as aqueous or organic extract of plants. Antioxidants, on interaction with DPPH, either transfer electrons or hydrogen atoms to DPPH, thus neutralizing the free radical character30. The degrees of discoloration caused by the loss of the initial purple showed the potential for binding of the free radicals. All extracts showed a considerable antioxidant potential (Table 2). The antioxidant activities of extracts of W. saharae leaves and the standard compounds were as follows:
Vitamin C > BHT > Vitamin E > Saponins extract > Ethanolic extract > Ethyl acetate extract. It’s reported that phenolic compounds have received considerable attention, due to their antioxidant activities and free radical scavenging abilities, which potentially have beneficial implications in human health31,32. As cited in many studies, natural antioxidants that are present in herbs and spices are responsible for inhibiting or preventing the deleterious consequences of oxidative stress33. The activities of natural antioxidants in influencing diseases are closely related to their ability to reduce DNA damage, mutagenesis, carcinogenesis and inhibition of pathogenic bacterial growth34.
Evaluation of anti-inflammatory effects showed that ethanolic extract of W. saharae decreased significantly the carrageenaninduced increase in paw diameter as compared with control rats.
As cited previously, leaves of W. saharae yielded sesquiterpene lactones of the guainolide group. Hilmi et al5 studied in parallel, the anti-inflammatory and the cytotoxic potential of the isolated guaianolides. Their results revealed that guaianolide type sesquiterpene lactones can differentially modulate gene transcription of pro-inflammatory and house-keeping genes despite of similar activity in NF-kB EMSA, IL-6 reporter gene, and cytotoxicity assays. In our best knowledge, no study has been conducted on experimental animals for the scientific evaluation of anti-inflammatory effects and toxicity on Warionia genus. The biochemical results were confirmed by the histological pattern of intact structure of liver tissues and normal kidneys showing normal tubular brush borders and intact glomeruli for the doses lower than 2.5 g/kg BW compared to the control group. While toxic sings were shown in the renal and liver tissues on treatment with dose 5 g/kg BW of ethanolic extract of W. saharae. Serum creatinine concentration was significantly increased (p < 0.05) in the treated group of animals (1.25, 2.5 and 5 g/kg BW) indicating the induction of nephrotoxicity when compared to the control rats. Hepatic function has been monitored by the evaluation of serum level of transaminases ALT and AST. The rise in serum levels of AST, ALT and cholesterol could been attributed to the damaged structural integrity of the liver, because they are cytoplasmic in location and released into circulation after cellular damages36.
CONCLUSION
The present study supported the utilization of W. saharae in traditional Moroccan medicine. The ethanolic extract of leaves has an anti-inflammatory activity. This effect could be due to the secondary metabolites by the same or different mechanisms of sesquiterpene lactones as showed by Hilmi et al5. The results indicated that ehtanloic extract did not produce histological toxic manifestations in rats in doses up to 2.5 g/kg BW and suggest that this plant present interesting potential source of natural phenolics and antioxidants that could be used to treat inflammatory diseases and to reduce oxidative stress in human beings.
REFERENCES
1. Nagore DH, Ghosh VK, Patil MJ, Wahile AM. In vitro antioxidant and in-vitro anti-inflammatory activity of Cassia sophera Linn. Int J Pharm Pharm Sci 2010;2: 113-121.
2. Bellakhdar J, Baayaoui A, Kazdari A, Marechal J. Herboristes et médecine traditionnelle à Tissint, oasis presaharien du sud Marocain (province de Tata). Al Biruniya 1986;3: 7-50.
3. Watillon C, Gaspar T, Hofinger M, Ramaut JL. La micropropagation de Warionia saharae Benth. & Coss. Al Biruniya 1987;4: 35-38.
4. Hilmi F, Sticher O, Heilmann J. New cytotoxic 6,7-cis and 6,7-trans configured guaianolides from Warionia saharae. J Nat Prod 2002;65: 523-526.
5. Hilmi F, Gertsch J, Bremner P, Valovic S, Heinrich M, Sticher O, et al. Cytotoxic versus anti-inflammatory effects in HeLa, Jurkat T and human peripheral blood cells caused by guaianolide-type sesquiterpene lactones. Bioorg Med Chem 2003a;11: 3659-3663.
6. Hilmi F, Sticher O, Heilmann J. New cytotoxic sesquiterpene lactones from Warionia saharae. Planta Medica 2003b;69: 462-464.
7. Essaqui A, Elamrani A, Benaissa M, Rodrigues AI, Yoongho L. Chemical composition of the leaves extract of Warionia saharae of Morocco. JEOBP 2004;7: 250-254.
8. Ramaut JL, Hofimger M, Dimbi R, Corvisier M, Lewalle J. Main constituents of the essential oil of Warionia saharae Benth & Coss. Chromatographia 1985;20: 193-194.
9. Essaqui A, Elamrani A, Cayuela JA, Benaissa M. Chemical composition of the Essential Oil of Warionia saharae from Morocco. JEOBP 2007;10: 241-246.
10. Znini M, Majidi L, Laghchimi A, Paolini J, Hammouti B, Costa J, et al. Chemical composition and anticorrosive activity of Warionia saharea essential oil against the corrosion of mild steel in 0.5 M H2SO4. Int J Electrochem Sci 2011;6: 5940-5955.
11. Amezouar F, Badri W, Hsaine M, Bourhim N, Fougrach H.
Chemical composition, antioxidant and antibacterial activities of leaves essential oil and ethanolic extract of Moroccan Warionia saharae Benth. & Coss. JAPS 2012;2: 212-217.
12. Katinas L, Tellería MC, Susanna A, Ortiz S. Warionia (Asteraceae): a relict genus of Cichorieae? Anal Jard Bot Mad 2008;65: 367-381.
13. Bremer K. Asteraceae: Cladistics and classification. Portland: Timber Press; 1994.
14. Wagner H, Bladt S, Zagainski EM. Plant drug analysis. Springer-Verlag: Berlin, Heidelberg, New York, Tokyo; 1984.
15. Trease GE, Evans WC. Trease and Evans Pharmacognosy. 15th ed. WB. Saunders Edinburgh London, New York: Philadelphia St. Louis Sydney Toronto; 2002.
16. Cannell RJP. Natural Products Isolation (Methods in Biotechnology). Cannell RJP ed. Humana Press, Totowa, New Jersey (USA); 1998.
17. Barbehenn RV. Measurement of protein in whole plant samples with ninhydrin. J Sci Food Agric 1995;69: 353-359.
18. Applebaum SW, Marco S, Birk Y. Saponins as possible factors of resistance of legume seeds to the attack of insects. J Agric Food Chem 1969;17: 618-622.
19. Chitindingue K, Ndhala AR, Chapano C, Benhura MA, Muchuweti M. Phenolic compound content, profiles and antioxidant activities of Amaranthus hybridus (pigweed), brachiaria brizantha (upright brachiaria) and Panicum maximum (guinea grass). J Food Biochem 2007;31: 206-216
20. Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem 2003;51: 609-614.
21. Zhishen J, Mengcheng T, Jiannming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999;64: 555-559.
22. Pothitirat W, Chomnawang MT, Supabphol R, Gritsanapan W. Composition of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the Mangosteen fuit rind at tow stages of maturity. Fitoterapia 2009;80: 442-447.
23. Prieto P, Pineda M, Aguillar M. Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenum complex: specific application to determination of vitamin E. Anal Biochem 1999;269: 337-341.
24. Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 1986;44: 307-315.
25. Winter CA, Risley EA, Nuss GW. Carrageenan-induced oedema in the hind paw of rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 1962;111: 544-547.
26. Adeyemi OO, Okpo SO, Oguni OO. Analgesic and anti-inflammatory effect of the aqueous extract of leaves of Persea Americana Mill (Lawraceae). Fitoterapia 2002;73: 375-380.
27. Lanhers MC, Fleurentin J, Rolland A, Vinche A. Activité Antiinflammatoire d'un extrait de Peumus Boldus Molina (Monimiaceae), Phytotherapy 1992; no38-39: 12-13.
28. Rached W, Benamar H, Bennaceur M, Marouf A. Screening of the Antioxidant potential of some algerian indigenous plants. J Biol Sci 2010;10: 316-324.
29. Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica 1982;45: 31-34.
30. Archana B, Dasgupta N, De B. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem 2005;90: 727-733.
31. Imeh U, Khokhar S. Distribution of conjugated and free phenols in fruits: Antioxidant activity and cultivar variations. J Agric Food Chem 2002;50: 6301-6306.
32. López-Vélez M, Martinez-Martinez F, Del Valle-Ribes C. The study of phenolic compounds as natural antioxidants in wine. Crit Rev Food Sci Nutr 2003;43: 233-244.
33. Acharya S, Sahu AR, Satyajit, Mohanta SR. Free radical scavenging activity of thalamus of Nymphacea stellata Willd. Int J Pharm Pharm Sci 2010;2: 61-63.
34. Roginsky V, Lissi EA. Review of methods to determine chainbreaking antioxidant activity in food. Food Chem 2005;92: 235-254.
35. Sallie R, Tredger JM, William R. Drugs and the liver. Part I. “Testing liver function”. Biopharmaceutics et drug disposition 1991;12: 251-259.
Source web: Prof. FATIMA AMEZOUAR, Prof. WADI BADRI1, Prof. MOHAMMED HSAINE, Prof. MOHAMMED AKSIM, Prof. NOUREDDINE BOURHIM, Prof. HASSAN FOUGRACH
Les tags en relation
Dictionnaire scientifique
Plus de 123.000 mots scientifiques
Les publications
Géo parc Jbel Bani

Circuits & excursions touristiques

cartothéques


Photothéques
Publications & éditions